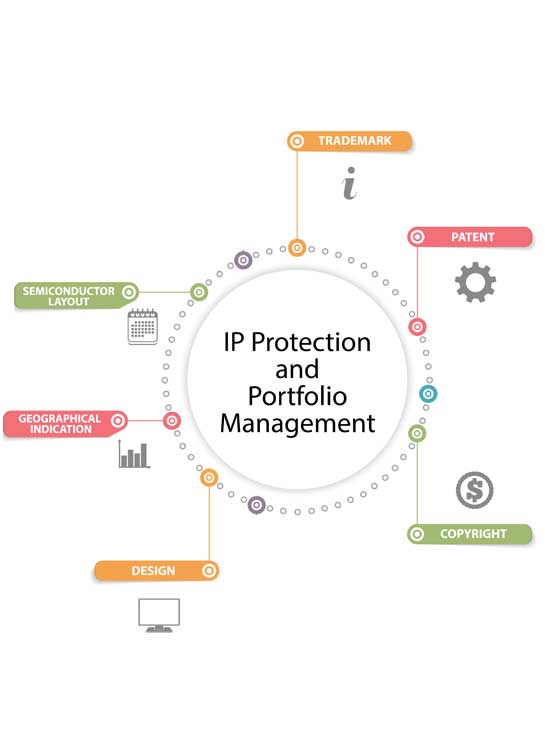



Khurana & Khurana has been established precisely to help corporate leverage anticipated business and economic value from its efficient creation, protection and enforcement of their Intellectual Properties. Khurana & Khurana has created a unique and strategic position for itself by giving End-to-End IP services to its clients from the inception stage of creation of IP to successful commercialization of the IP for fulfillment of desired business objectives. The role and importance of patent professionals in IP (intellectual property) portfolio management (IPM) are increasing becoming significant within business, academic, and legal entities.
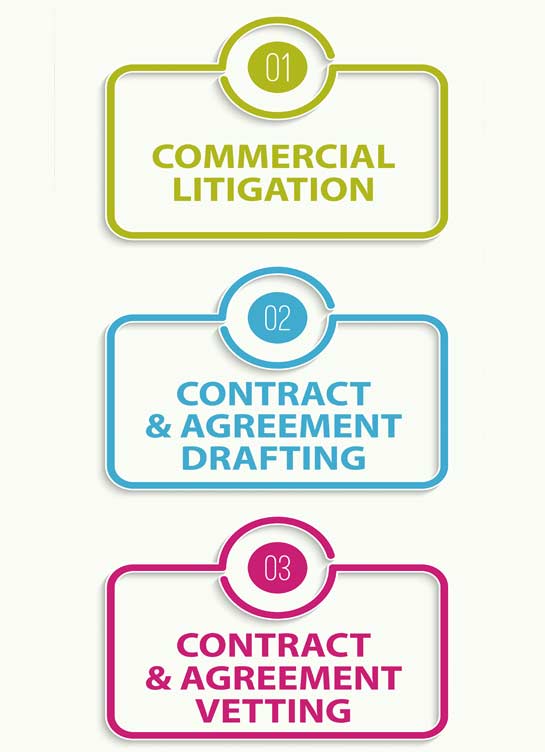
View moreKhurana & Khurana has a legacy of over ten years in Patent Litigation and serves some of the leading Corporates for IP infringement matters. Our experience includes finding the potential infringers for the IP owners through due diligence and assessing the market for the same. For doing this, our firm has Patent/Trade Mark Attorneys/Practitioners who have advanced qualifications in varied technology domains and understand complete nuances of claim mapping and provisions of enforcement under different IP Laws.
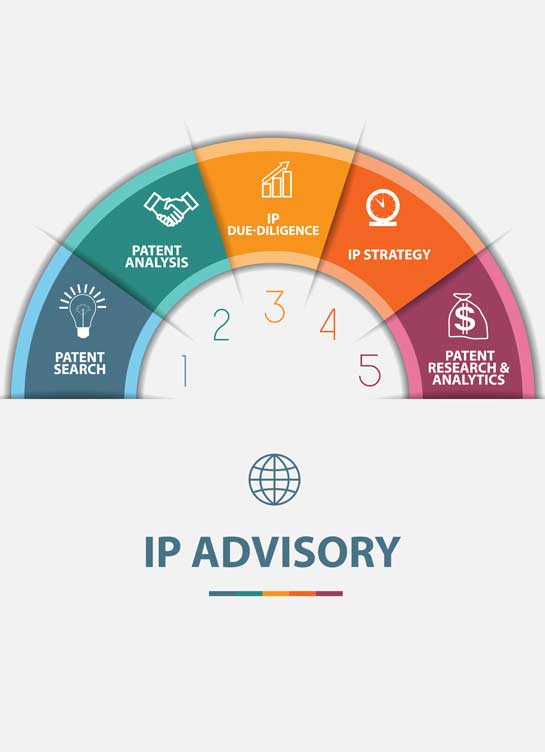
View moreKhurana & Khurana assists Corporates in maximizing opportunities from their IP portfolios through accurate legal opinions that ensure that all possible ways to solve a problem are identified and due-diligence of each way is put across in the right manner such that the client can take a judicious call on the way to proceed forward. K&K renders Prior art, Freedom to Operate, Validity, and Non-infringement opinions relating to a client’s products and technologies. K&K also prepares a comprehensive IP Litigation Strategy before initiating with a legal suit to ensure that the clients understands the next steps clearly, sets its expectations, and is cognizant of the costs involved in the process.
View moreCommercial law governs the broad areas of business, commerce and consumer transactions. Commercial law in India has developed rapidly over the years with the opening up approach towards FDI and WTO. It allows, so far as it can, Corporate or person to do business in the way they want and not require them to stick to forms that they may think to be outmoded. Thus, Commercial Law Practice is recognized as a very important and integral element of any corporate operation, and is gaining more and more importance.In this era of globalisation, sweeping changes in business strategies require Corporates to meet challenge of complying with commercial contract law for the smooth functioning of its business and commerce.
View moreThe Internet Corporation for Assigned Names and Numbers (ICANN) is responsible for the administration of Top Level Domain Names. The Uniform Dispute Resolution Policy provides for the settlement of domain name disputes for the Generic Top Level Domains Name (GTLD) such as .com, .edu, .net, among others. The UDRP complaints may be filed with any of the ICANN accredited arbitration centres. UDRP also allows for the filing of the complaints at the jurisdictional civil court where the Registrant or the Registrar is located. Where the Registrar or the Registrant is located in India, the dispute may be resolved through legal action at the civil court.
View moreAttorneys at Khurana & Khurana have developed significant experience in the area of anti-counterfeiting, especially in FMCG, Pharmaceuticals, Apparels, Footwear, and Medical Devices product verticals, and can assist in devising a comprehensive strategy for curbing counterfeit products by outlining all possible risks and assumptions and mapping them with costs involved in each step taken so that a judicious and objective step can be taken by the client..
View more
Published on 27th April, 2024
INTRODUCTION The regulation of assisted reproductive technologies (ART) is central to the complex interplay between ethics and law. The Assisted Reproductive Technology (ART) Regulation Act, focusing on Indian context, is a key determinant of reproductive autonomy through legislation especially for women. Besides establishing regulations to ensure that ART clinics are properly functioning and protect women … Continue reading Traversing the Ethical and Legal Maze: Overview of the Art Regulation Act, 2021
Read More
Published on 26th April, 2024
This article refers to copyright and patent to be granted in case of confidential information and the requirements with respect to the same in order to prove liability. It also refers to various obligations that arise once a party is exposed to some confidential information and the remedies that are available. However, there is a … Continue reading Copyright for Confidential Information
Read More
Published on 25th April, 2024
Abstract Idea expression is the fundamental principle of copyright. This is also given in TRIPS, WIPO Copyright Treaty. Through this article, we will discuss the concept of Idea expression dichotomy, merger doctrine, and Scenes a Faire. We will also discuss the reasoning of the court while deciding the matter of idea-expression dichotomy. We will see … Continue reading Idea-Expression Dichotomy in Copyright: Judicial Rulings and Merger Doctrine
Read More
Published on 24th April, 2024
Abstract– article 370 which provides special status to the state of Jammu and Kashmir has been a topic od debate for a long time. On August 2019, the Indian government repealed article 370 which was a groundbreaking decision which basically means taking out all the special rights and privileges which was given under article 370 … Continue reading Decoding the Abrogation of Article 370- Impact & Debates
Read More
Published on 23rd April, 2024
INTRODUCTION Corporate governance is how a corporation’s values, guiding principles, and management practices are ingrained and manifested. Corporations, governments, investors, and others now acknowledge that diversity on company boards is critical to successful corporate governance. “Diversity in boardrooms” is a hotly contested issue in the field of corporate governance nowadays. Economic growth depends on empowering women to … Continue reading Women Directors and Their Position in India
Read More
Published on 22nd April, 2024
INTRODUCTION TO THE PERSONAL LAWS India’s personal law is diverse because of its many historical influences, unique guiding principles, and the vast majority of substantive law. Broadly there are four different religions in our country that is Hindu, Muslim, Parsi, Christian and they are governed by distinct laws. A variety of succession laws are lawfully … Continue reading Fundamental Differences Hindu & Muslim Law of Inheritance
Read More
Published on 19th April, 2024
“The police are prosecuting on the effect of the deep fake and not because it is a deep fake itself” According to policy researchers at Digital Futures Lab in Goa, Indian regulations do not currently provide a precise definition of “deepfakes” The Information Technology Act and laws prohibiting defamation, fake news and invasions of privacy … Continue reading The Use Of Deep Fake
Read More
Published on 18th April, 2024
Most of the survey takers belonged to the age of 20 years i.e., 38.5%, which is equal to 45 individuals. The second highest survey takers belonged to the age group of 19 years i.e., 32.5%, which is equal to 38 individuals. The individuals of 14 years, 15 years, 16 years, 17 years, 18 years who … Continue reading Period Poverty and Law: An Emperical Research – Part II
Read More
Published on 17th April, 2024
INTRODUCTION During the catastrophic and unprecedented situation during the Covid-19 pandemic in the year 2020, the people encountered the longest and most stringent lockdown at the national level. Though the lockdown was imposed with an aim to benefit the people at a large, it resulted in the destruction of the global economy, earnings of daily-wage … Continue reading Period Poverty And Law: An Empirical Research
Read More
Published on 16th April, 2024
Introduction India has witnessed exponential growth in e-commerce and online market place in the last few years. Customers are now able to have a totally different and new shopping experience, as the E-Commerce system makes it easy for consumers to trade goods and services with the help of technology, pricing, attractive discounts, and fast delivery … Continue reading Trademark Infringement in E-Commerce in India: Challenges in Digital Era
Read More
Published on 10th April, 2024
Abstract A pristine environment is fundamental for sustaining life on Earth. Early human societies thrived with abundant natural resources and a profound reverence for the environment, including rivers, mountains, trees, and plants. However, on December 2nd, 1984, the tranquil town of Bhopal was thrust into chaos due to a catastrophic incident at Union Carbide Plant … Continue reading Legal Considerations of Air Pollution in Light of the Bhopal Gas Disaster
Read More
Published on 9th April, 2024
Introduction The Competition Act[1] describes ‘turnover’ as the ‘value of sale of goods or services’. Associated with this provision, is the provision specifying the penalty[2] for anti-competitive agreements,[3] which until recently, did not specify whether this ‘turnover’ was to be based on total/global turnover (derived from all the products and services by an enterprise)[4] or … Continue reading From ‘Relevant’ To ‘Global’ Turnover: Examining The Basis Of Penalty Under The Competition (Amendment) Act, 2023
Read More